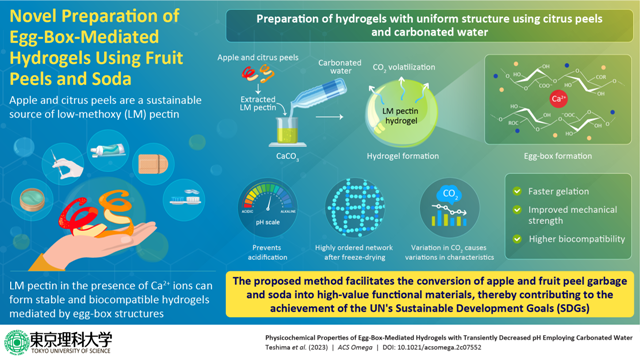
Introducing carbonated water into a mixture of low-methoxy pectin and calcium carbonate solution can accelerate the gelation process, improve the mechanical strength of hydrogels, and induce intrinsic structural changes in the gel material—found a recent Tokyo University of Science research. The insights from this study validate the use of carbonated water as a means of supplying CO2 and controlling the properties of hydrogels.
Low-methoxy (LM) pectin gels have gained momentum in tissue engineering and drug delivery in recent years due to their excellent biocompatibility. LM pectin forms stable hydrogels by forming supramolecular assemblies with egg-box structures in the presence of divalent ions like Ca2+, generally provided by a calcium chloride (CaCl2) solution or calcium carbonate (CaCO3). In particular, the use of CaCO3 enables the preparation of LM pectin gels with controlled shape. Previous research has shown that minute changes in hydrogel synthesis precursor parameters can majorly impact the hydrogel properties and their applications. For instance, decreasing the pH or acidifying the precursor accelerates the gelation rate and increases the mechanical strength of LM pectin hydrogels. CO2 is a popular acidic agent because it can decrease pH and be easily removed from the system after gelation. However, despite CO2 being a promising acidic agent, a precise understanding of its effects on hydrogel synthesis and properties is poorly understood.
A group of scientists from Japan, led by Mr. Ryota Teshima, a Master’s student from Tokyo University of Science (TUS), decided to fill this gap. To do so, they prepared LM pectin/CaCO3 hydrogels using the standard method and under controlled thermodynamic conditions with carbonated water as the source of CO2. The research team also included Dr. Shigehito Osawa (currently at Tokyo Women’s Medical University), and Dr. Yayoi Kawano, Prof. Takehisa Hanawa, Prof. Akihiko Kikuchi, and Prof. Hidenori Otsuka from TUS. Their research was made available online on February 16, 2023, and published in volume 8, issue 8 of ACS Omega on February 28, 2023.
Explaining the reasoning behind this approach, Mr. Teshima says, “We chose carbonated water as the acidic agent for gelation because conventional methods would require high-pressure conditions to ensure proper supply of CO2 to the hydrogel precursor. Instead, using carbonated water allowed us to supply carbonate ions and CO2 to the hydrogel mixture and trigger gelation under ambient pressure and temperature conditions.”
The team simultaneously prepared two LM pectin/CaCO3 hydrogels—one with added carbonated water and the other without. They observed that the one with carbonated water underwent faster gelation and showed increased mechanical strength compared to the one without. These improvements could arise from the lower pH of carbonated water enhancing the solubility of CaCO3, which further increases the availability of free Ca2+ ions. More Ca2+ means better cross-linking between pectin polymers, quicker gelation, and more robust hydrogels.
After the hydrogel formation, carbonated water/CO2 was volatilized from the surface in contact with the atmosphere. The team found that post-CO2 escape, the pH of the final hydrogel was higher than the hydrogel prepared without carbonated water. The team suggested this could be because many carboxy groups were consumed during cross-linking. with Ca2+ and the acidic component, CO2, was completely volatilized from the hydrogels after gelation. The team further prepared aerogels by freeze-drying the hydrogels. They found highly ordered networks of elongated porosity in those prepared through the carbonated water route, suggesting that the CO2 from carbonated water could induce intrinsic structural changes in the hydrogels. The researchers also prepared hydrogels with different amounts of CO2 in the carbonated water to demonstrate that the pH and strength of LM pectin hydrogels can be tuned in this manner. The insights from this study can prove helpful when fabricating functional hydrogels with tailored properties.
This research contributes to the United Nations’ sustainable development goals (SDGs), as the LM pectin was primarily derived from food waste, such as apple and citrus fruits. “Every year, 2.5 billion tons of food is wasted worldwide, which includes vegetables and fruits that are discarded without ever reaching the market because of quality issues. We believe that the promising results of our study will encourage other researchers to use items thrown out as food waste as raw materials for their scientific ventures,” concludes Mr. Teshima.